Bubble wrap
What is the problem with plastic?
In the middle of the XIX century, the colonial economies had an environmental disaster on their hands. Even worse, they also had an economic problem. Ivory could not be found easily on the market. The price was skyrocketing, and demand far surpassed supply. Ivory is a bony substance found in the tusks of elephants and walruses, and in the horns of rhinos and narwhals. It is made from dentine and collagen, the main components of mammal teeth, and it has been heavily appreciated as a material for ornaments and decorations throughout most of history. Entire societies in the medieval era were supported by the production of this material. For example, the vikings in Greenland that lived by exporting ivory, among other products, to pay for iron, wood and even food between the X and XV centuries.
But coming back to the 1800s, ivory was running out.
Narwhals and walruses did not offer large amounts of the material and had been extensively hunted. Rhinos were not exactly easy to hunt. The majority of the ivory was produced with elephant tusks, and they were slowly going extinct before they were even coming close to quenching the thirst for their tusks. One example of an object made from ivory that was essential for the British colonial empire was billiard balls. Ivory was considered as the only possible material for them: no other material even approached the desired properties for a smooth game of pool. A solution was needed to this dire situation.
It is just at this moment that plastics were invented.
A gentleman called Alexander Parkes found out the first chemical synthetic method for cellulose nitrate. This is the first manufactured material in history that we can properly call a plastic product, and its creator called it Parkesine. The discovery was serendipitous. This material had the perfect physical properties to substitute the role that ivory had in society. It was just as good or better to the eyes and to the touch, it could be manufactured from agricultural waste products for a tiny cost, and there was no limitation to the supply in sight. Others took his invention and ran with it, making the first commercial plastic products. It was a sign of progress, of technology improving the lives of everyone and lifting us to a better society.
Why is it that nowadays, more than a century later, we seem to have a problem with plastics?
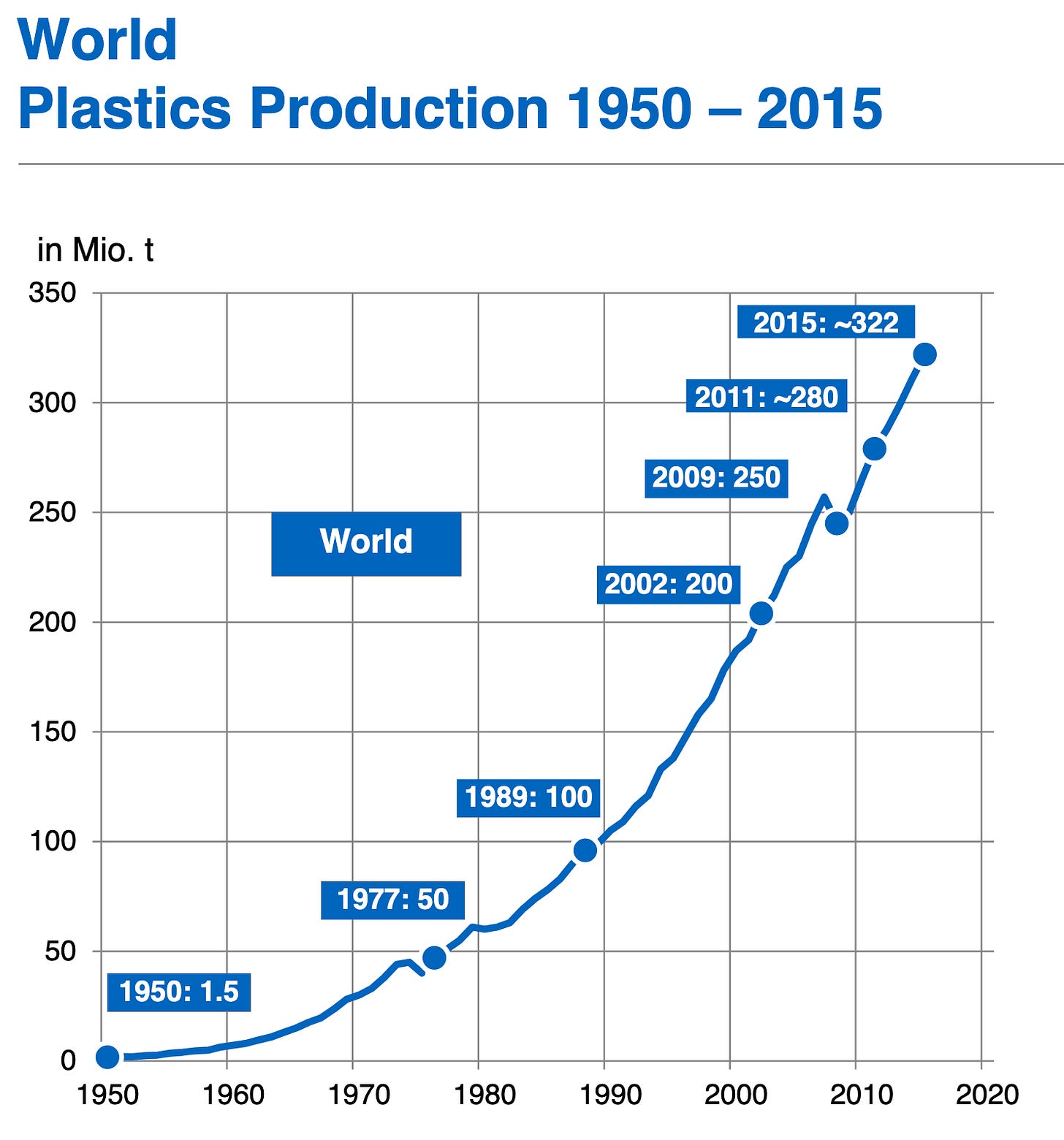
The answer to that question is not nearly as straightforward as it sounds. Yes, nowadays plastics are associated with cheap, disposable wrappings that end up in the natural ecosystems somehow, destroying nature. But in the 20th century, plastics meant higher quality objects, stronger materials and richer families. Nearly every household in industrialised countries contained some plastic products, although at that moment these materials were used for high quality commodities, like radioes, vinyls or cinema rolls. Over time, new types of plastics were developed and used in all kinds of purposes, from military equipment to furniture to plastic bags and water bottles. Plastic waste did not become a problem to take into account until the post-WW2 era, when mass production started for consumer goods and specially single-use plastics. The explosion in use and disposal was beyond anything the industry titans and governments expected, and it quickly overloaded the very limited waste management solutions available at that moment.
“Eternal materials that do not degrade or corrode and last forever” has a nice ring to it, very much in line with the technological optimism of the time. The implications of this eternal life was clearly not in the mind of public officials or the public. A common explanation for why this was the case is that nobody knew better, that there was no plan nor alternative, and that the solutions were improvised as soon as the problems were found. We are trapped in a mire of plastic today, due to short-sightedness, bad management and unforeseen consequences.
Except that is very much not what happened. It is demonstrably false that nobody knew this was going to become a problem, and in fact there were a few loud voices pointing out what we consider today as common sense: that plastics are not going to go anywhere if we do not take care of them proactively. Chemical engineers, the very same creators of all kinds and varieties of plastics, were the first ones to notice the consequences of their creation and ring the alarm. We can see dozens of examples of scientists, engineers and other technically-minded people that wrote repeatedly about this topic when plastics have been in common circulation in consumer products for less than 20 years.
Plastics cannot and will not degrade and reintegrate to the natural environment in productive ways, at least not in short enough amounts of time. They are remarkably resilient to any conditions we expose them to, and they will accumulate in the natural environment.
The chemical engineers understood the chemical nature of the material, the fact that it would never go away on its own, contrary to virtually every other material used by humanity. The fact that it would enter natural ecosystems and just keep circulating on and on and on, with likely unforeseen consequences from this chemical and physical invasion. It was a crisis, and treated as such, in a very similar language to the one we use today.
Regretfully, they were ignored. Or no actionable solution was found to be satisfactory at that time. Recycling was elevated as the hope for the future, the only possible solution to the avalanche of plastic that we did not know what to do about. Possibly they were flippant and overconfident in their abilities. Maybe they had not yet tried a lot of different approaches and failed, like we do now. This article is a story about chemistry, about engineers being ignored and entrenched problems. Most of all, it is a story that proves the consequences of not dealing with our waste. We drown in plastic, even when we don’t have to.
Why plastic recycling is a fantasy
In order to illustrate the problem, let us follow the life cycle of a regular water bottle. Such a bottle is normally composed of polyethylene terephthalate, also known as PET. This is a thermoplastic polymer. Thermoplastic means that this material is easy to mold when heated, and polymer means that the material is composed of a chain of monomers, units of a simpler molecule. In fact, the defining characteristic of plastics is that they are polymers. The composition, length, additives and other parameters of the polymer is what determines its physico-chemical properties, thus allowing us to tailor plastics to whichever application we desire.
Single use plastic bottles tend to be made from PET because it is lightweight, strong and easy to mold in different shapes according to the size, brand or aesthetics required. It is biologically innocuous to animals, insoluble in water and nearly impossible to tear down by mistake. All of these properties make PET an excellent plastic for the storage and transport of food and water for long periods of time, especially if the consumption date is years away from its manufacture. Moreover, PET can do this work alone, without needing any other molecule in the mix, but it can also be mixed with other plastics and other materials to obtain varied and improved characteristics for different purposes. The units that compose the chain are based on ethylene glycol and terephthalic acid, both of which are ultimately produced from hydrocarbons. In the past, oil was the most common hydrocarbon used as a source, but nowadays methane is also used in large quantities by most refinery ecosystems. It’s all very old biomass, in any case.
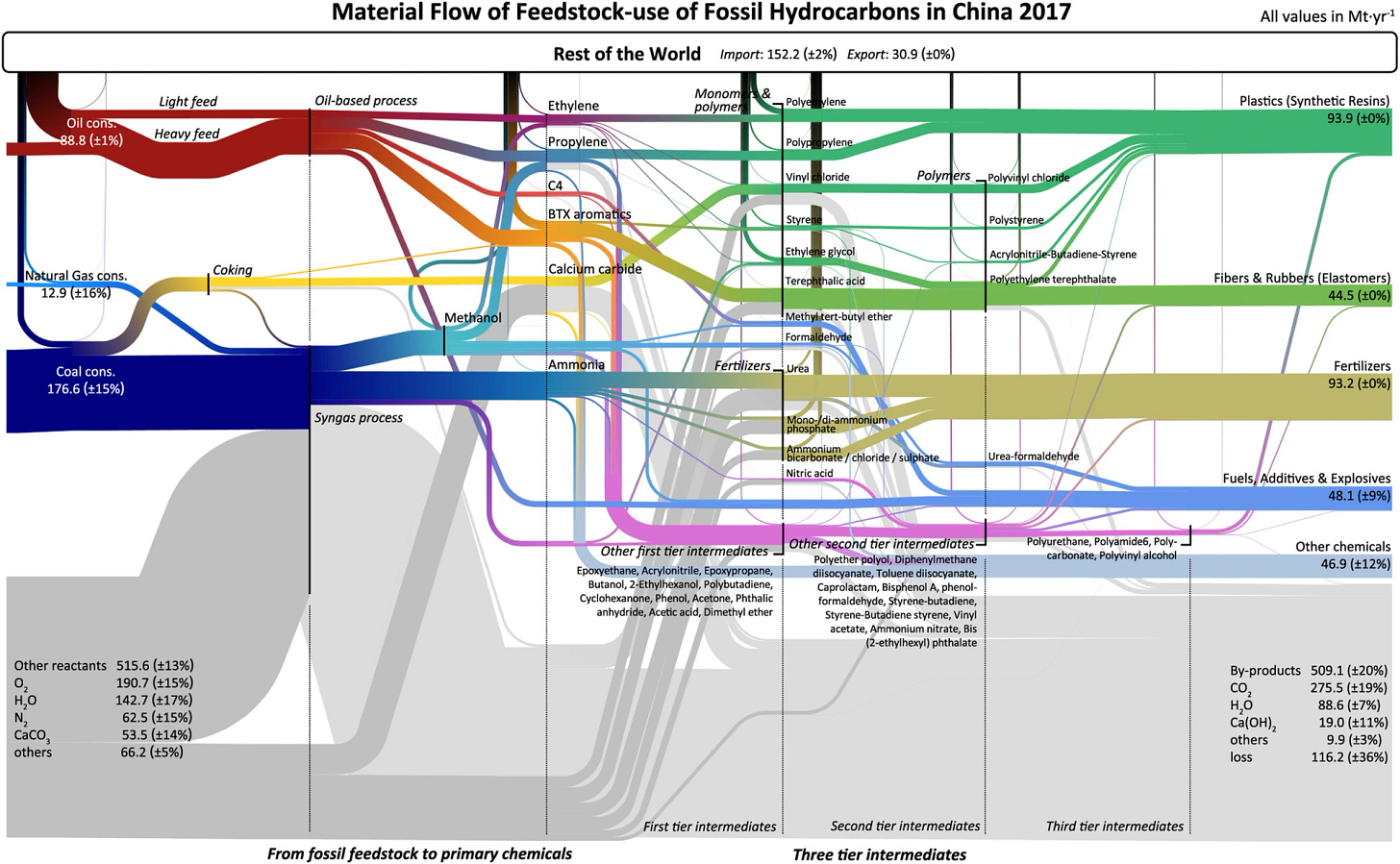
Specifically, in the Chinese hydrocarbon refinery ecosystem petrochemicals are mostly derived from coal turned into methane and then into plastics, while in the American one they are directly derived from natural gas derived from fracking. Other regions and countries use whichever mix is available for them; they are fundamentally derived from the cheapest source, whichever that happens to be. From Jiang et al, 2024.
When we dispose of this plastic bottle in a bin, its chemical composition matters a lot to what happens next. Since it is made from PET, that makes it recyclable. PET waste can be used to produce new PET objects. For the most part. Actually, this is a bit misleading. PET is a polymer after all. When it was produced, it was produced to a particular length, the molecules had a specific number of monomers per chain that gave it part of its amazing properties.
The recycling process consists essentially of separating PET by color (to also prevent ugly color mixing), grinding the plastic in small pieces for easier handling, cleaning any organic contaminant and melting it to be reformed in pellets that can later be fed to molding machines as recycled plastics. The melting process is not perfect for PET, it does not just turn the material liquid, but also partially degrade it, it cuts some monomers from the chains that are released as volatiles and gases like CO2. The recycled PET has shorter chains, weaker links, worse properties than what is usually called virgin plastic. There is no reverse reaction to enlarge the chains again, and there is no direct solution. Recycled plastic and virgin plastic are mixed together to minimize the damage done by this degraded material, and even then a small reduction in quality is possible depending on how much of each plastic is used. But it does work, some of the plastic is recycled and the rest escapes to the atmosphere as degradation products. Problem solved.
Except plastic bottles have caps. Which are not made from PET, but from polypropylene (PP), another polymer. I will not enter why different plastics are used, but normally each plastic has been carefully chosen for its specific properties in their ability to perform a function, and any other choice tends to be worse or totally unviable. We have to deal with the cap, it is mandatory to separate the different plastics to recycle them. If we melt them together, it is as impossible to separate them without destroying the material as it would be to separate water and apple juice mixed together: we can separate the simplest components, but there is no way to get a fresh apple at the end. Thankfully, PP is also a thermoplastic polymer, and it happens to float in water while PET does not, so after grinding you can separate caps and bottles in a big bathtub, and do a similar recycling step for each of them, with consequent degradation. All is well still.
Except there is the label too. Labels tend to be made of plastic film of different kinds, like PE, PET, PVC, PP or BoPP. We need to confirm the composition after we take the label, and classify it accordingly for recycling. Some of these are entirely unrecyclable too, or need a totally different method other than melting. But now we are finished and have all components separated and classified, we can recycle by material. Or we can use solvents to dissolve the adhesives, and separate again by flotation, at the cost of contaminating our PP stream.
Except there are plenty of bottles that are not based on a single polymer, but on a mixture of them. Maybe 90% PET 10% PP, or some other mixture deemed necessary to contain a particular liquid. We are back to having a mixed soup of degraded plastics, so we need to invest a lot of money and several years researching a specific recycling method based on some other physical or chemical principle for this particular mixture. It is costly but doable.
But most countries do not separate plastic bottles (save honorary exceptions like Germany and USA). Most countries have a totally nonsensical trash sorting method, from the point of view of recycling and reuse (link previous article). Most plastics end up in mixed waste containers. That includes paper, metal and plastic contaminated by organic matter that used to be contained in it, at random concentrations that can hardly be predicted.
I will be perfectly blunt: it is totally impossible to recycle a mixed waste bin. It is not a matter of difficulty, cost or will, it is just technically impossible to do at any reasonable cost. It is chemically impossible, like reversing entropy in the universe. It is in fact reversing entropy that we attempt to do when we recycle, and we can only do it when it is not too far gone. There is no connection whatsoever between our waste collection system and our recycling system, thus most of our waste will never be recycled no matter what engineers do. And plastics are always in the mixed waste. This is not by mistake though. Plastics are intrinsically mixed materials, by definition and by chemistry. Polymers, remember?
The next impulse one feels is to try and improve our waste collection system, or even our waste generation. Let us legally control the types of plastics that can be used, preventing the mixing at the source. Let us prevent factories from making products that have pieces of different types of plastic. We shall separate each waste by their chemical composition, so let us train every single person in how to recognize and classify the materials, and set steep fines to promote sorting at the household level. Each human produces on average 0.78 kg of trash a day, about 285 kg per year per person, so you surely need to recruit everyone into this project.
Obviously, I am joking. There is nothing doable about taking the necessary steps to make our plastic streams fully recyclable. Every year we produce millions of tons of PET, but we can barely recover a fraction of that in the best of cases. And so far we are talking exclusively about PET, the most well understood example for plastic recycling. Every other molecule is going to be orders of magnitude harder to process. The benefits are tiny compared to the costs of such a task, we definitely should not try to recycle all of our plastics. It is a foolish idea from beginning to end, if you understand a bit of chemistry.
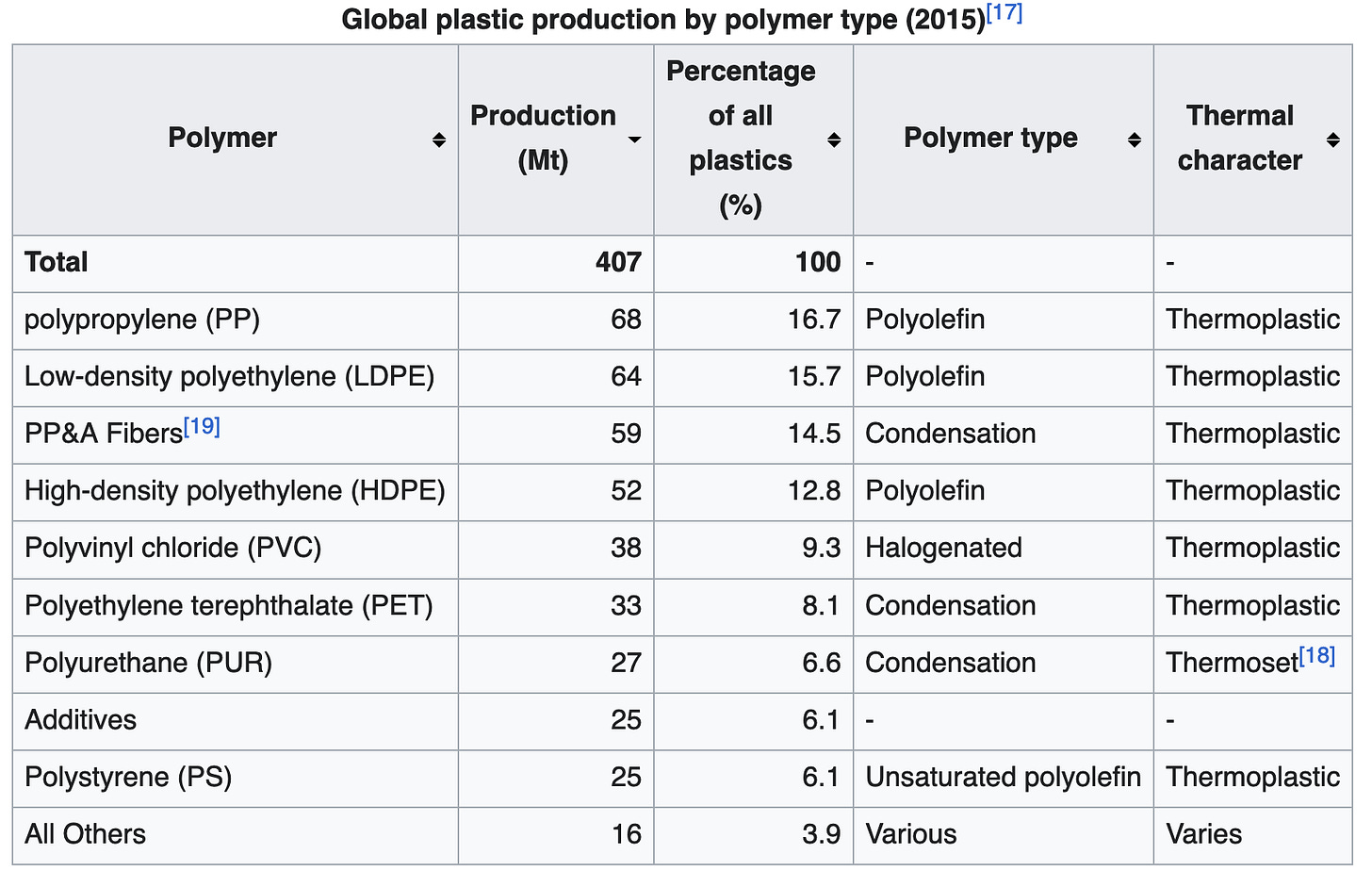
Plastics are classified by numbers, one to seven, as shown in the image below. PET, HDPE, LDPE and PP are highly recyclable plastics, and virtually all of the recycling efforts you have heard of in the news are focused on this subset of plastics, and relatively high rates of recycling are achieved by separating the material at the source whenever possible. Every other plastic material is virtually unusable. Note that 7 includes the vast majority of polymers in existence, and even when measured by mass, the recyclable plastics only account for about 50% of yearly production.
From Wikipedia.
Plastics always create mixed, non recyclable waste.
Of course, chemical engineers knew this. And they were vocal about it, last century. Everyone with a minimal knowledge of chemistry that hears about this problem can understand how recycling is just unworkable under the current conditions, and nearly impossible even in the most optimal scenario.
The issue is that all of those that tried to raise the alarm were plainly ignored. They were ignored because this is a very inconvenient truth.
Inconvenient to plastic producers, true, as they are the most directly benefited by the production and sale of plastics. But the rest of us are to blame too.The group most inconvenienced by this truth is the general public, the consumer. The consumer does not like to minimize its own plastic use. The consumer likes to have safe food products that last for ages in the pantry, and that means plastics. The consumer likes cheap furniture from IKEA. The consumer wants to have cheap knick knacks available, cheap clothes from Shein. The consumer wants its deliveries to be shielded by a protective layer of bubble wrap, keeping everything inside safe. The consumer wants to hear a story of circular flow of materials, of spring renewal from the ashes, no matter how nonsense or anti-scientific that is as a concept. The consumer blames all the plastic pollution on the chemical companies, while it buys and buys and wants to be protected by the bubble wrap too. The consumer just wants the plastic waste problem to simply disappear without them lifting a finger.
Our standard of living would not be nearly as high without plastic materials, not by a long shot. We would be measurably poorer without them. And yet, they cause problems. Problems that we need to acknowledge to even attempt to solve them. Right now, the story of plastic waste is the story of mixed, non recyclable trash. This is not a cyclical story, but a spiral one. An uncontrolled spiral of unbalanced material production that should accumulate exponentially, make piles that can be seen over the horizon like mountains, as we have learned before. But look outside your window. Where is it? Across most of the industrialized countries you can hardly see any trash, anywhere. You introduce it in a bin, and it vanishes like smoke. Except it is obviously not disappearing, a trashman took it. Took it somewhere. Ask yourself.
Where is all the trash going?
References
The Age of Plastic: From Parkesine to Pollution
Science Museum. (2019, October 11). The Age of Plastic: From Parkesine to pollution. Retrieved 23.05.2025, from https://www.sciencemuseum.org.uk/objects-and-stories/chemistry/age-plastic-parkesine-pollution
The Plastic Industry (Berlin, August 2016)
PlasticsEurope Market Research Group & Consultic Marketing & Industrieberatung GmbH. (2016, August 20). The Plastic Industry (Presentation). In ISO/TC 61 Plastics (Berlin, August 2016). Retrieved 23.05.2025, from https://committee.iso.org/files/live/sites/tc61/files/The%20Plastic%20Industry%20Berlin%20Aug%202016%20-%20Copy.pdf
Breidenbach, A. W. (1970). Needs for Chemical Research in Solid Waste Management. US Department of Health, Education, and Welfare, Public Health Service, Environmental Health Service, Bureau of Solid Waste Management.
Tracing Fossil-Based Plastics, Chemicals and Fertilizers Production in China
Jiang, M., Cao, Y., Liu, C., Chen, D., Zhou, W., Wen, Q., ... & Zhu, B. (2024). Tracing fossil-based plastics, chemicals and fertilizers production in China. Nature Communications, 15(1), 3854.
How Does PET Plastic Recycling Work?
Recycle the One. (n.d.). How does PET plastic recycling work? Retrieved 23.05.2025, from https://www.recycletheone.com/recycling-pet-plastic/how-does-pet-plastic-recycling-work/
Material Recycling of Plastics—A Challenge for Sustainability
Lahl, U., & Zeschmar-Lahl, B. (2024). Material Recycling of Plastics—A Challenge for Sustainability. Sustainability, 16(15), 6630.
Plastic – Wikipedia (Classifications Section)
Wikipedia contributors. (n.d.). Plastic – classifications. In Wikipedia. Retrieved 23.05.2025, from https://en.wikipedia.org/wiki/Plastic#Classifications